 How a spectrometer works
How a spectrometer works
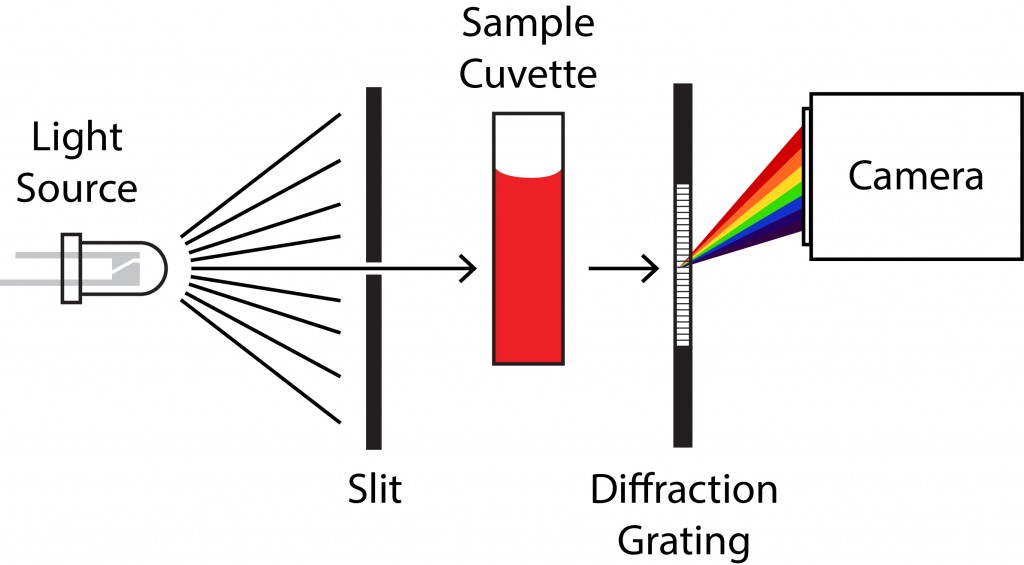
A spectrometer is nothing more than a device to split light into its different colors (a prism or a diffraction grating) that projects onto a camera (usually a CCD chip). Since different wavelengths of light deflect by different amounts, each wavelength arrives at a unique location on the camera. The correspondence between wavelength and pixel position is built into the spectrometer’s software, which displays the total intensity (the reading at the appropriate pixel on the camera) as the amount of light at that wavelength.
 You can see the beam path in the actual workings of our spectrometer. The CCD chip is on the left. It looks like the spectrometer uses a prism (located near the center of the board) to split light coming from the cuvette holder (on the right).
You can see the beam path in the actual workings of our spectrometer. The CCD chip is on the left. It looks like the spectrometer uses a prism (located near the center of the board) to split light coming from the cuvette holder (on the right).
Using our spectrometer
We will use our spectrometer in three modes:
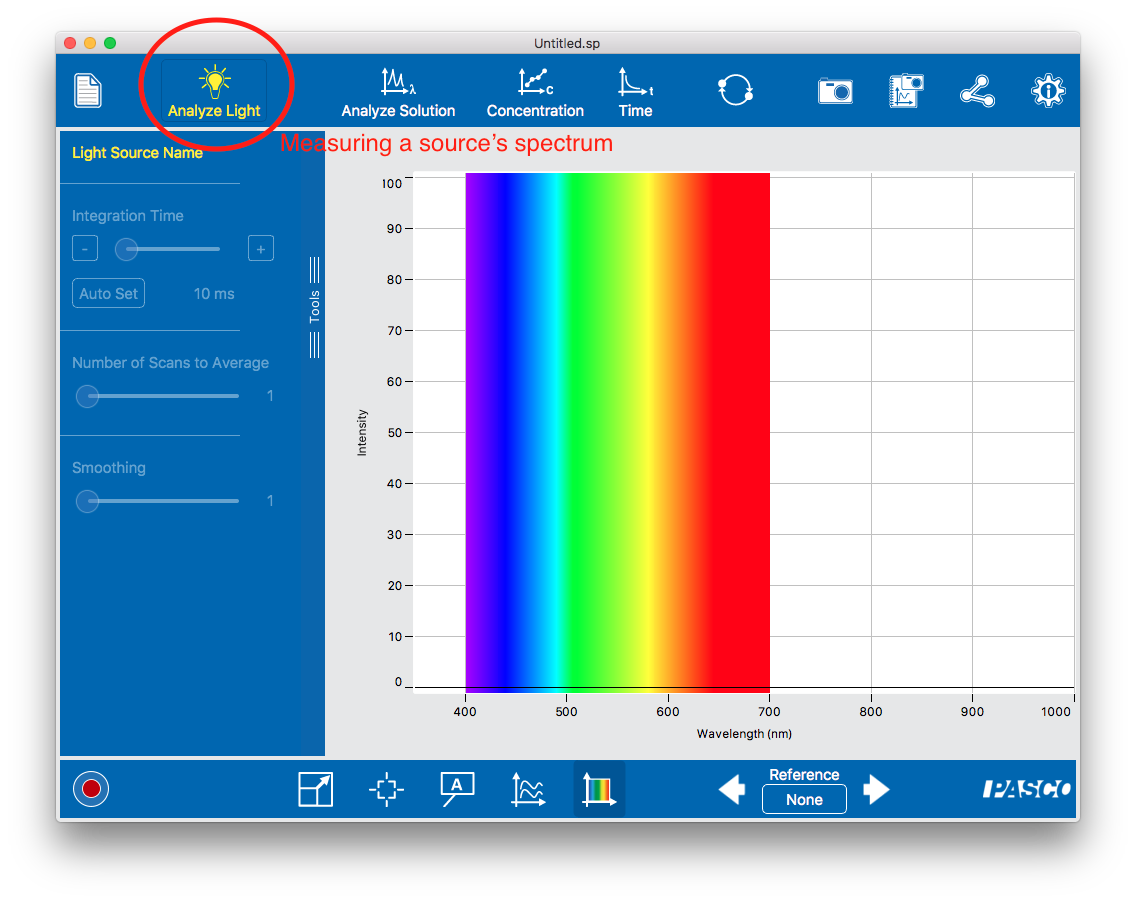 To measure the spectrum of a light source. Use the fiber optic insert (a fake cuvette with an optical fiber attached to it) and point the end of the cable at the source. Light is piped directly into the spectrometer’s optical path and the spectrometer measures and displays the intensity.
To measure the spectrum of a light source. Use the fiber optic insert (a fake cuvette with an optical fiber attached to it) and point the end of the cable at the source. Light is piped directly into the spectrometer’s optical path and the spectrometer measures and displays the intensity.- To measure the absorbance of a sample. White light shines through a sample cuvette; some is absorbed and never makes it to the CCD chip. The spectrometer measures the amount of light (as a function of wavelength) relative to a blank (a transparent sample) and displays either the amount transmitted (as a percentage) or the absorption (defined as the base logarithm of the ratio of absorbed to reference light). In this mode you will need to take a dark measurement (no light) and a reference measurements (with a transparent cuvette in the spectrometer).
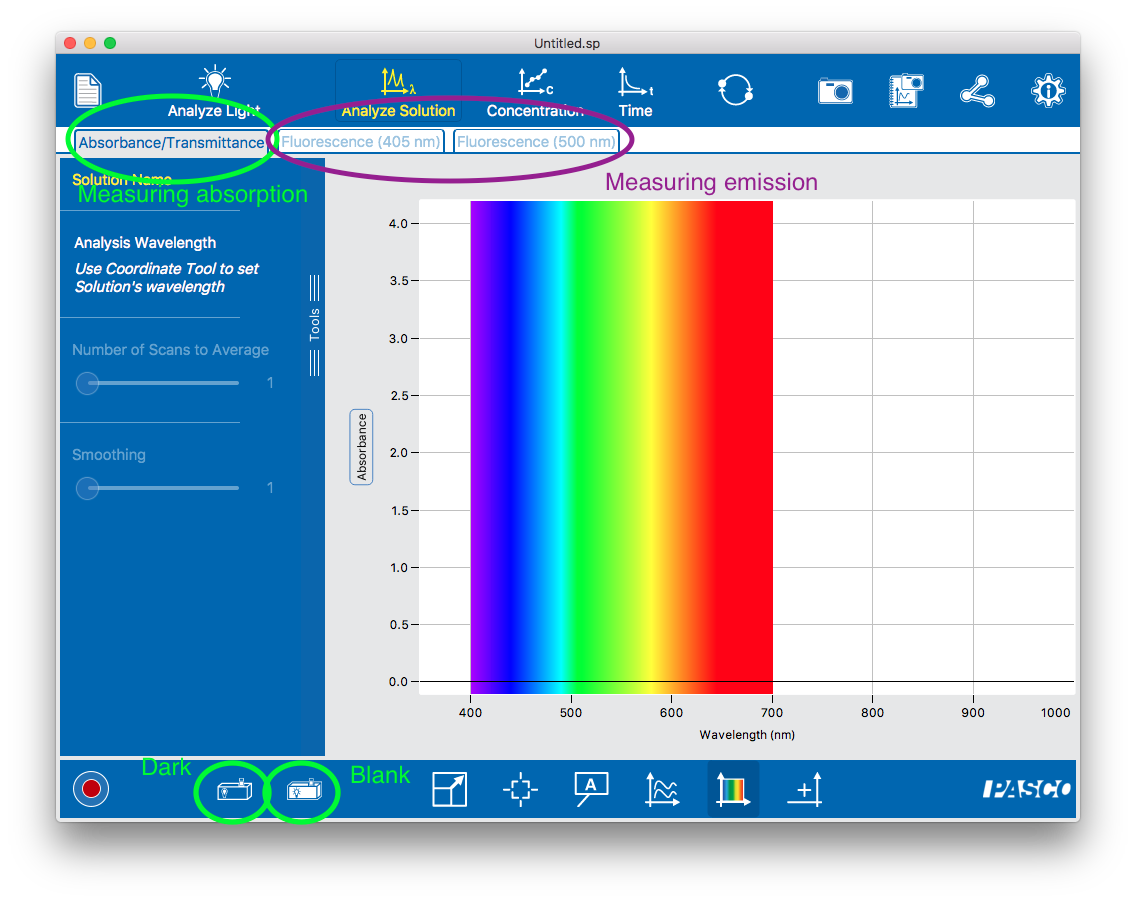 To measure the fluorescent emission of a sample. Violet or green excitation light shines on the sample; light that is emitted at right angles to the excitation light is measured by the spectrometer and the intensity is displayed.
To measure the fluorescent emission of a sample. Violet or green excitation light shines on the sample; light that is emitted at right angles to the excitation light is measured by the spectrometer and the intensity is displayed.
The spectrometer software is available here. You will need to go through a cumbersome registration, though, so you may want to grab the software directly for Mac or for PC from me.
Technical details about absorption measurements
The amount of incident light that a sample absorbs is called its absorption. There are two ways to report absorption: Transmittance (the % of light that is not absorbed) and Absorbance (a logarithmic measure of the fraction of light that is not absorbed; this is also called optical density). Our spectrometer has a single “absorption” mode (which is somewhat confusingly labeled “Absorbance / Transmittance”) and you switch between Absorbance and Transmittance units by clicking on the y axis of the plot),
In Absorbance / Transmittance mode, you will need to first take “Dark” and “Blank” measurements. You should be prompted to do this, or you can proactively take (or retake) these using the two icons in the lower left corner of the Absorbance / Transmittance screen (circled in the figure). This is necessary because absorption is relative: since it quantifies the fraction of original light that is absorbed by the sample, it first needs to measure the original light intensity (the blank). The blank should be recorded under the same conditions as the sample (same cuvette, same solvent, same light source) except that it doesn’t contain the compound of interest. This way any reduction in light intensity is solely due to the compound’s absorption.
Mathematical definitions of T and A
If the spectrometer records an intensity I0 from the blank and I from the sample, then
- The transmittance T is defined as T = 100 (I/I0). So 80% transmittance means that 80% of the original (blank) light still gets through the sample; only 20% of the light is absorbed.
- Absorbance is defined as A = -log10(I/I0). The negative sign makes absorbances be positive numbers, which I guess are more convenient to work with (since we always have I < I0, log10(I/I0) < 0). An absorbance of 1 means that I/I0 = 0.1: 90% of the original (blank) light is absorbed; only 10% of the light gets through.
Additionally, for technical reasons the spectrometer also takes a “dark” reading with the light turned off. In principle this ought be zero, but in practice real instruments have a nonzero background. (You can see that this value is nonzero if you record the light spectrum with your hand over the empty cuvette well.) This is subtracted from the actual instrument reading: in reality, I = IMeasured – IDark.